LIAISON® Parvovirus B19 Diagnostic Solution
Parvovirus (Fifth disease) can have deadly consequences for the unborn fetus during the pregnancy period.
Screening during pregnancy can significantly reduce this risk. The LIAISON® Parvovirus B19 assays panel (IgG, IgM) is a complete automated solution to support clinicians for differential diagnosis of Human Parvovirus B19 infection.
LIAISON® Biotrin Parvovirus B19 IgG
LIAISON® Parvovirus B19 IgG is a CLIA assay for the qualitative determination of specific IgG antibodies to Human Parvovirus B19 in human serum or plasma samples.
LIAISON® Biotrin Parvovirus B19 IgG Plus
LIAISON® Parvovirus B19 IgG is a CLIA assay for the quantitative determination of specific IgG antibodies to Human Parvovirus B19 in human serum or plasma samples.*
*Product availibility subjet to regulatory approval. Please consult your local Diasorin team.
LIAISON® Biotrin Parvovirus B19 IgM Plus
LIAISON® Parvovirus B19 IgM is a CLIA assay for the quantitative determination of specific IgM antibodies to Human Parvovirus B19 in human serum or plasma samples.*
*Product availibility subjet to regulatory approval. Please consult your local Diasorin team.
LIAISON® Biotrin Parvovirus B19 IgM
LIAISON® Parvovirus B19 IgM is a CLIA assay for the qualitative determination of specific IgM antibodies to Human Parvovirus B19 in human serum or plasma samples.

Background
Parvovirus B19 is an erythrovirus that has been associated with a growing number of clinical presentations, the two most important being Fifth Disease in children and fetal complications during pregnancy. Observed in outbreaks, Parvovirus B19 is highly contagious and is associated with fever, muscle pains, arthralgia and a characteristic facial rash. In pregnancy, infection of the fetus during the first 28 weeks of term can lead to cardiac failure, spontaneous abortion, or fetal hydrops.
How it works
Disease evolution
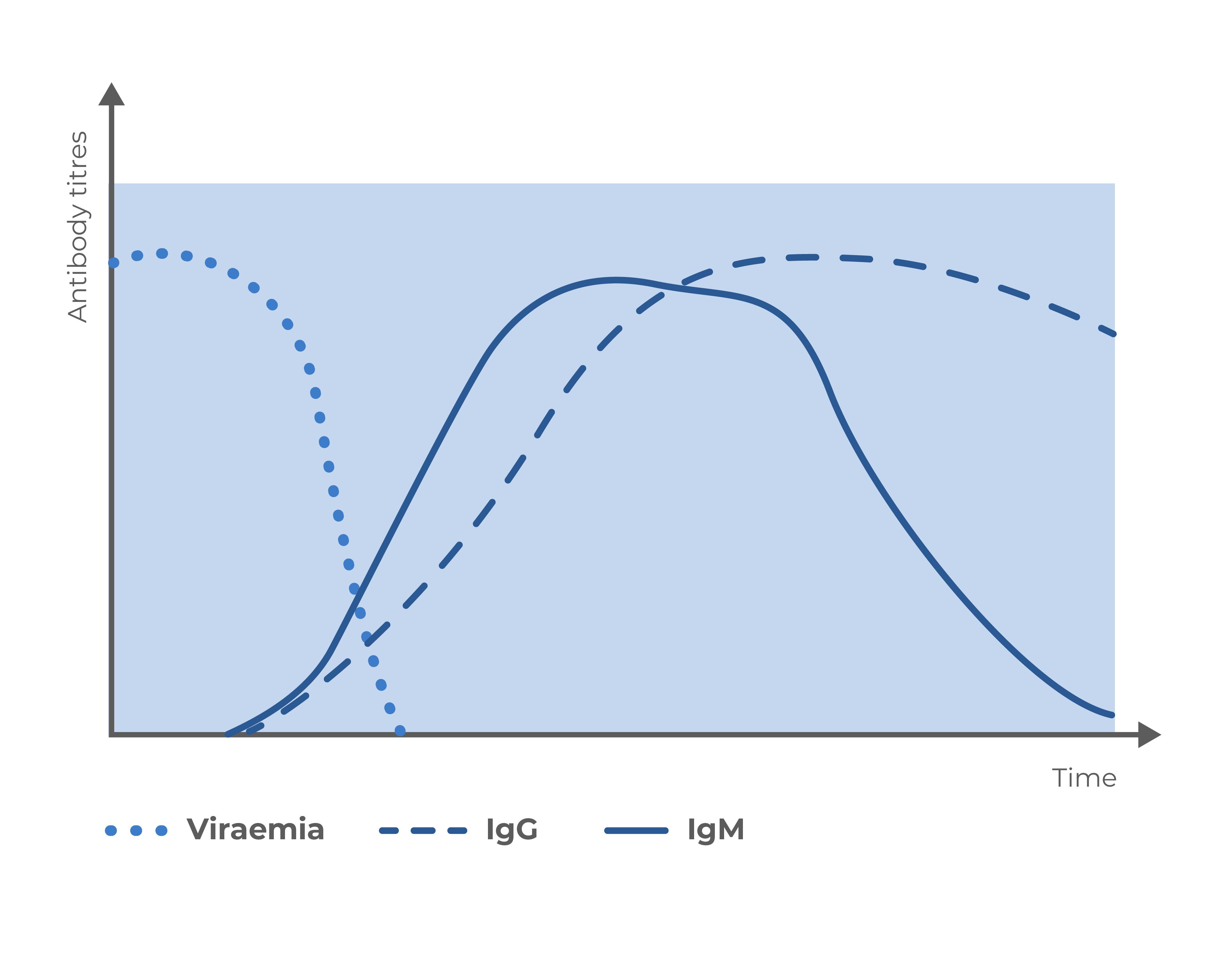
Results interpretation
IgG |
IgM |
Diagnostic |
|---|---|---|
| Negative |
Negative |
No infection |
| Positive |
Negative |
Past infection |
| Negative |
Positive |
Acute infection |
| Positive |
Positive |
Acute infection |
Why use LIAISON® Parvovirus B19 Diagnostic Solution
The combined use of LIAISON® Biotrin Parvovirus B19 IgG and IgM can aid the diagnosis of Fifth disease.
LIAISON® Biotrin Parvovirus
B19 IgM
The detection of IgM antibodies will alert the clinician to a current Parvovirus B19 infection.
LIAISON® Biotrin Parvovirus
B19 IgG
Screening for IgG antibodies to Parvovirus B19 allows the assessment of immune protection in pregnant woman.
LIAISON® Biotrin Parvovirus
B19 IgM Plus
The detection of IgM antibodies will alert the clinician to a current Parvovirus B19 infection.
LIAISON® Biotrin Parvovirus
B19 IgG Plus
Quantitative results standardized vs WHO second international standard NIBSC 01/602.

To be used on
Designed for both specialty and routine tests, LIAISON® XL and LIAISON® XS immunoassay analyzers help your laboratory handle multiple patients and tests simultaneously. LIAISON® systems are trustworthy, intuitive and deliver automated continuous operation with minimal user intervention. The result is reduced turnaround time, optimal cost management and unmatched growth potential.

LIAISON® XL
Designed for large laboratories. Fuse the benefits of high throughput and high sensitivity within a powerful and fully automated system that can seamlessly connect to facilitate Total Laboratory Automation.

LIAISON® XS
A fully automated, easy-to-use benchtop analyzer. Maximize productivity with optimal cost management, no daily maintenance, straightforward integration, and the same capabilities as Diasorin’s high-throughput analyzers.
More details about our diagnostic solutions
Login to Dialog for additional resources
Login to our repository for instructions for use and user manuals, assay information, protocols and much more.
