LIAISON® Toxoplasma gondii Diagnostic Solution
The diagnosis of primary Toxoplasma gondii infection in pregnant women and determining the infection timing are key in managing risk to the fetus.
The LIAISON® Toxoplasma assay panel (IgG, IgM, IgG Avidity) is a complete solution to support clinicians in detecting and dating the infection.
LIAISON® Toxo IgG II
LIAISON® Toxo IgG II is a CLIA assay for the quantitative determination of specific IgG antibodies to Toxoplasma gondii in human serum or plasma samples.
LIAISON® Toxo IgM
LIAISON® Toxo IgM is a CLIA assay for the quantitative determination of specific IgM antibodies to Toxoplasma gondii in human serum or plasma samples.
LIAISON® Toxo IgG Avidity
LIAISON® Toxo IgG Avidity is a CLIA assay for the determination of antigen-binding avidity of IgG antibodies to Toxoplasma gondii in human serum or plasma samples.
Background
Toxoplasma infection can cause severe damage in cases of congenitally acquired infection. The diagnosis of primary infection in pregnant women and the timing of infection are of particular importance. Serology is the only method to determine if the mother has been infected by toxoplasma gondii. Early diagnosis of primary infection requires a highly sensitive and quantitative assay for IgG and IgM antibodies, to discriminate between chronic and recent infections. IgG avidity improves the accuracy of diagnosis, dating the infection more precisely.
How it works
Disease evolution
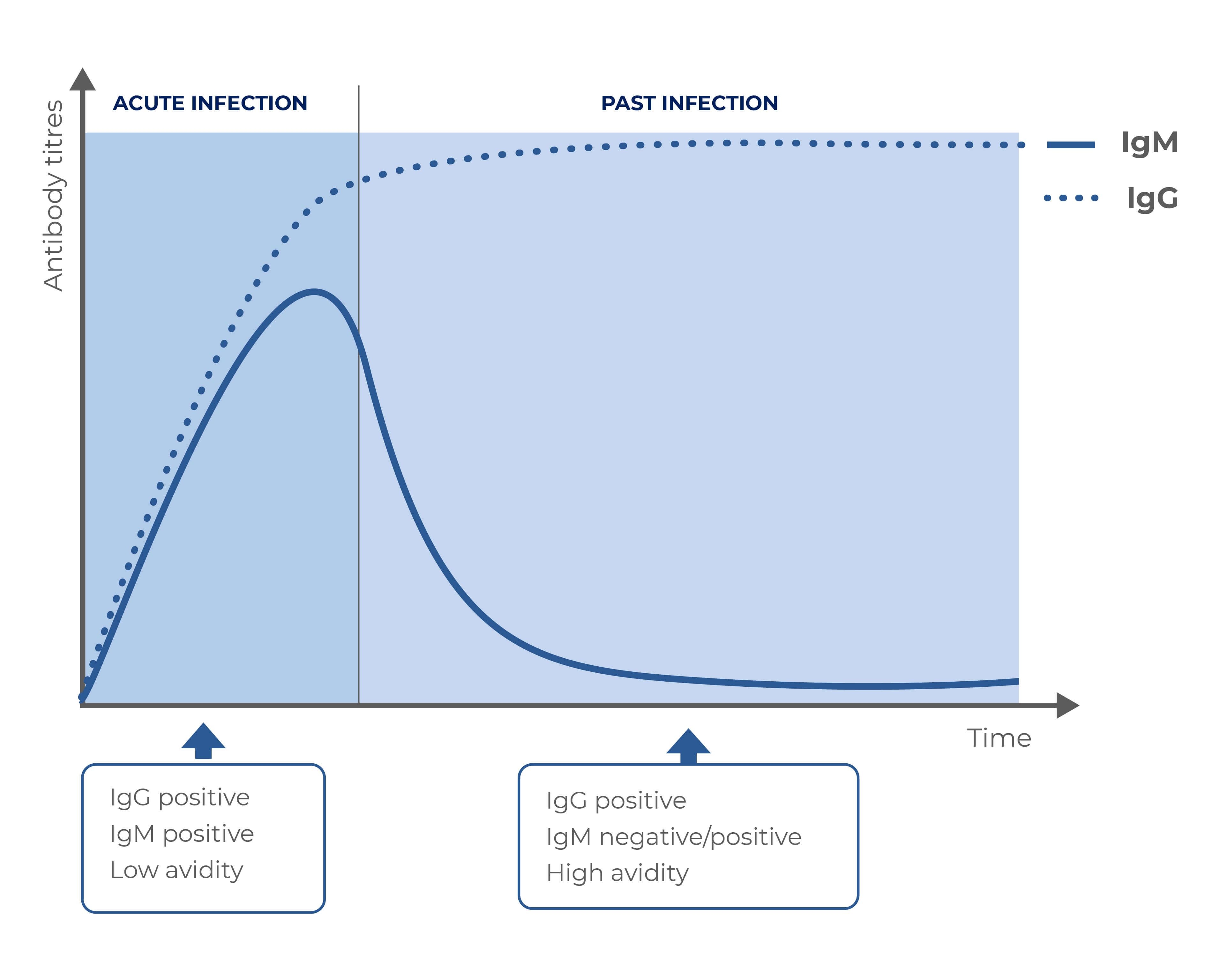
Results interpretation
IgG |
IgM |
Avidity |
Diagnosis |
|---|---|---|---|
| Positive |
Positive |
High |
Past infection |
| Negative |
Negative |
- |
No infection |
| Positive |
Negative |
- |
Past infection |
Why use LIAISON® Toxoplasma gondii Diagnostic Solution
The complete solution for Toxoplasma gondii serology, from detecting to dating the infection.
Detect acute infections
Measurement of IgG avidity may improve the accuracy of the serological diagnosis dating the infection more precisely. A high avidity index excludes recent toxoplasma infection within the last four months.
To improve the accuracy of the serological diagnosis, dating the infection more precisely
A high avidity index, determined by measuring IgG avidity, excludes recent toxoplasma infection within the last four months.

To be used on
Designed for both specialty and routine tests, LIAISON® XL and LIAISON® XS immunoassay analyzers help your laboratory handle multiple patients and tests simultaneously. LIAISON® systems are trustworthy, intuitive and deliver automated continuous operation with minimal user intervention. The result is reduced turnaround time, optimal cost management and unmatched growth potential.

LIAISON® XL
Designed for large laboratories. Fuse the benefits of high throughput and high sensitivity within a powerful and fully automated system that can seamlessly connect to facilitate Total Laboratory Automation.

LIAISON® XS
A fully automated, easy-to-use benchtop analyzer. Maximize productivity with optimal cost management, no daily maintenance, straightforward integration, and the same capabilities as Diasorin’s high-throughput analyzers.
More details about our diagnostic solutions
Login to Dialog for additional resources
Login to our repository for instructions for use and user manuals, assay information, protocols and much more.
