LIAISON® EBV Diagnostic Solution
Accurate differential diagnosis of the EBV stage of infection.
By providing a full panel for the parallel determination of VCA IgG, EBNA IgG and EBV IgM levels, Diasorin enables efficient detection of EBV infection and an optimized discrimination of the infection stage.
LIAISON® EBV IgM
CLIA assay for the quantitative determination of specific IgM antibodies to Epstein-Barr viral capsid antigens (VCA) in human serum or plasma samples.
LIAISON® VCA IgG
CLIA assay for the quantitative determination of specific IgG antibodies to Epstein-Barr viral capsid antigens (VCA) in human serum or plasma samples.
LIAISON® EBNA IgG
CLIA assay for the quantitative determination of specific IgG antibodies to Epstein-Barr virus nuclear antigen (EBNA) in human serum or plasma samples.
LIAISON® EA IgG
CLIA assay for the quantitative determination of specific IgG antibodies to Epstein-Barr virus early antigen-diffuse [EA(D)] in human serum or plasma samples.
Background
Epstein-Barr Virus (EBV) is the causative agent of infectious mononucleosis.
The clinical diagnosis can be challenging because the symptoms are similar to other illnesses and serological testing is used to exclude other diseases and assess disease states.
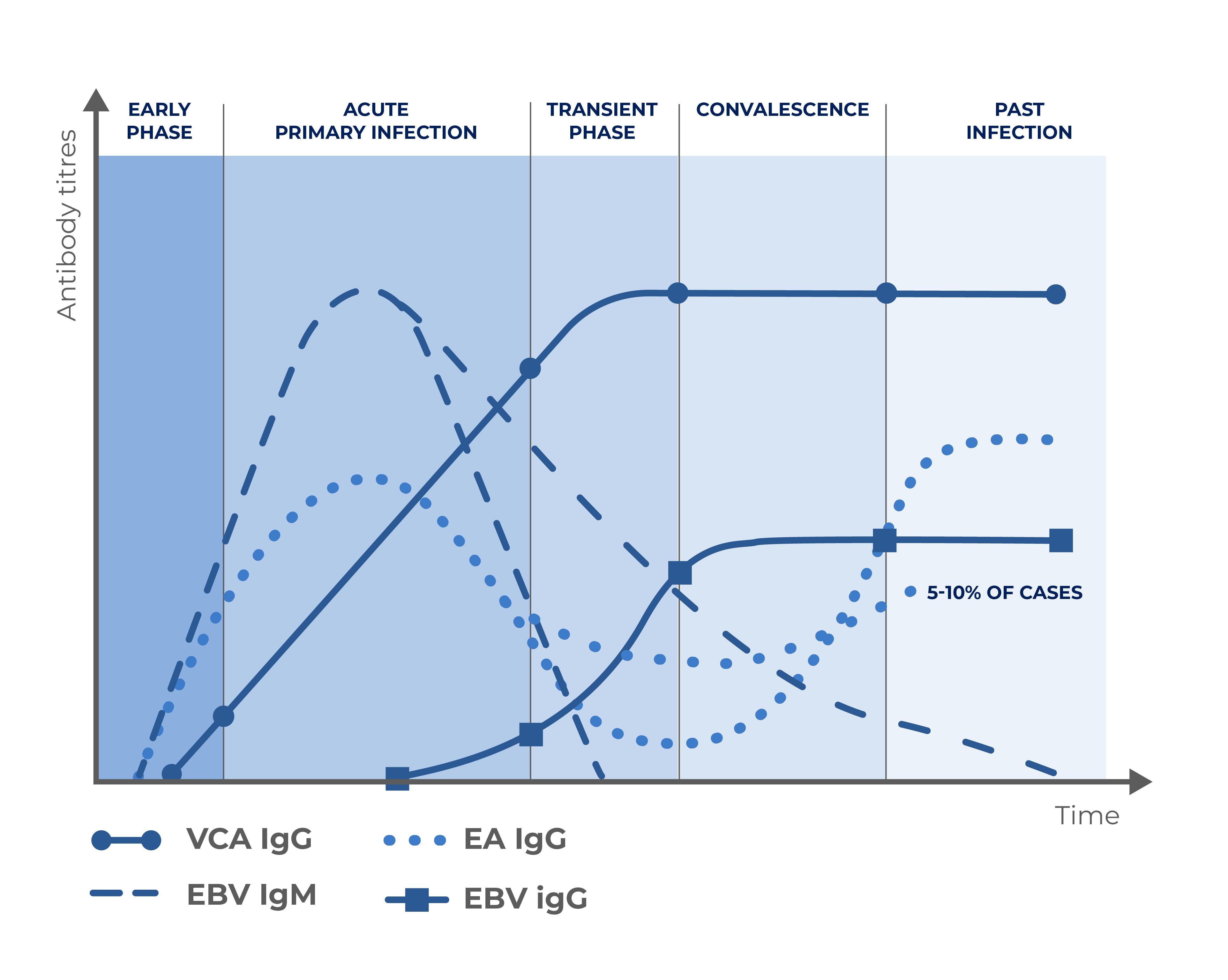
EBV serological testing measures IgG and IgM antibodies directed against different components of the virus. Antigens from the Viral Capsid (VCA) are used to detect antibodies generally produced during the acute phase of the infection whereas the EBNA-1 protein is used to detect IgG produced during convalescence.
Quantitative assays and use of a differential cut-off for the interpretation of EBNA IgG and EBV IgM results provide clinicians with better discrimination among different phases of EBV infection.
How it works
Disease evolution
Results interpretation
VCA IgM |
VCA IgG |
EBNA IgG |
EA IgG |
Diagnosis |
|---|---|---|---|---|
| - |
- |
- |
- |
EBV negative |
| + |
- |
- |
- |
Early phase of acute infection |
| + |
+ |
- |
+/- |
Acute primary infection |
| + |
+ |
+ |
+/- |
Transiente phase / reactivation |
| - |
+ |
+ |
+/- |
Past infection |

Why use LIAISON® EBV Diagnostic Solution
Fully automated EBV test panel aiding in the differential diagnosis and staging of EBV infection
Quantitative assays
Full panel of quantitative assays.
Differential cut-off
The use of a differential cut-off for the interpretation of EBNA IgG and EBV IgM results, allows better discrimination among different phases of EBV infection.
To be used on
Designed for both specialty and routine tests, LIAISON® XL and LIAISON® XS immunoassay analyzers help your laboratory handle multiple patients and tests simultaneously. LIAISON® systems are trustworthy, intuitive and deliver automated continuous operation with minimal user intervention. The result is reduced turnaround time, optimal cost management and unmatched growth potential.

LIAISON® XL
Designed for large laboratories. Fuse the benefits of high throughput and high sensitivity within a powerful and fully automated system that can seamlessly connect to facilitate Total Laboratory Automation.

LIAISON® XS
A fully automated, easy-to-use benchtop analyzer. Maximize productivity with optimal cost management, no daily maintenance, straightforward integration, and the same capabilities as Diasorin’s high-throughput analyzers.
More details about our diagnostic solutions
Login to Dialog for additional resources
Login to our repository for instructions for use and user manuals, assay information, protocols and much more.
